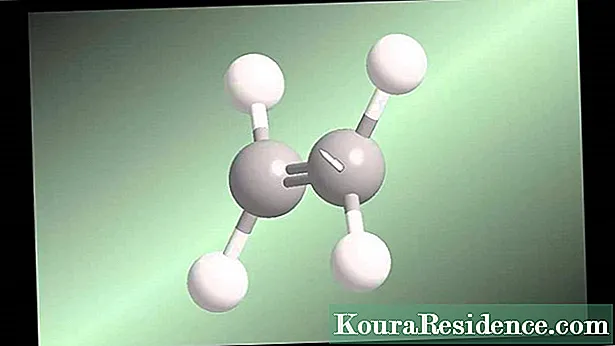
Ukwakha ama-molecule we izinhlanganisela zamakhemikhali, ama-athomu wezinto ezahlukahlukene noma izinto kufanele ahlangane ngendlela ezinzile, futhi lokhu kungenzeka ngezindlela ezahlukahlukene ngenxa yezici zokwakheka i-athomu ngayinye enayo, njengoba, njengoba sazi, iqukethe i-nucleus efakwe kahle ezungezwe yifu lama-electron.
Ama-electron akhokhiswa kabi futhi ahlala eseduze ne-nucleus ngoba amandla kagesi ibaheha. Lapho i-electron isondela ku-nucleus, kukhula amandla adingekayo ukuyikhulula.
Kepha akuzona zonke izinto ezifanayo: ezinye zinomkhuba wokulahlekelwa ama-elektroni angaphandle wefu (izinto ezinamandla aphansi e-ionization), kanti ezinye zivame ukuzibamba (izakhi ezinokuhlangana okuphezulu kwe-electron). Lokhu kwenzeka ngoba ngokusho komthetho ka-Lewis octet, ukuzinza kuhlotshaniswa nokuba khona kwama-electron ayi-8 kugobolondo elingaphandle noma ku-orbital, okungenani ezimweni eziningi.
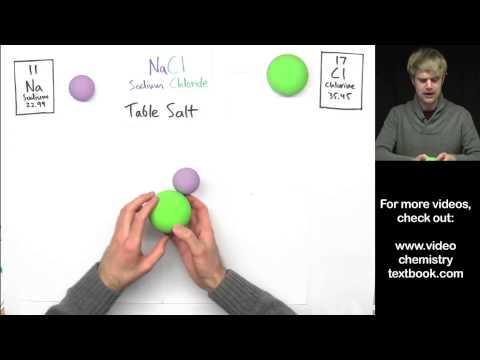
Ngemuva kwalokho kanjani kungaba nokulahleka noma ukuzuza kwama-electron, ama-ion wecala eliphikisanayo angakhiwa, futhi ukukhanga kwe-electrostatic phakathi kwama-ion wecala eliphikisanayo kwenza lezi zijoyine futhi zakhe izinhlanganisela zamakhemikhali ezilula, lapho esinye sezakhi sanikeza ama-electron kwathi esinye sawathola. Ukuze lokhu kwenzeke futhi a isibopho se-ionic kuyadingeka ukuthi kube nomehluko noma i-delta ye-electronegativity phakathi kwezinto ezithintekayo okungenani eziyi-1.7.
I- isibopho se-ionic imvamisa yenzeka phakathi kwenhlanganisela yensimbi kanye nenye engeyona eyensimbi: i-athomu yensimbi inika i-elektroni elilodwa noma amaningi futhi ngenxa yalokho yakha ama-ion (ama-cations) akhokhiswa kahle, kuthi i-nonmetal izizuze bese iba yizinhlayiyana ezikhokhiswe kabi (anion). Izinsimbi zomhlaba ze-alkali ne-alkaline yizakhi ezivame ukwakha ama-cations kakhulu, futhi ama-halogen ne-oxygen ngokuvamile ama-anion.
Ngenjwayelo, izinhlanganisela ezakhiwa yizibopho ze-ionic kukhona okuqinile ekamelweni lokushisa nasendaweni ephakeme yokuncibilika, encibilikayo emanzini. Esixazululweni kunjalo abaqhubi abahle bakagesinjengoba zingama-electrolyte aqinile. Amandla we-lattice we-ionic solid yiwo abonisa amandla ahehayo phakathi kwama-ion alokho okuqinile.
Ingakusebenzela:
- Izibonelo ze-Covalent Bonds
- Magnesium oxide (MgO)
- I-sulphate yethusi (CuSO4)
- I-potassium iodide (KI)
- Zinc hydroxide (Zn (OH) 2)
- I-sodium chloride (NaCl)
- I-nitrate yesiliva (AgNO3)
- I-lithium fluoride (LiF)
- Magnesium chloride (MgCl2)
- I-potassium hydroxide (KOH)
- I-calcium nitrate (Ca (NO3) 2)
- I-calcium phosphate (Ca3 (PO4) 2)
- I-potassium dichromate (K2Cr2O7)
- I-disodium phosphate (Na2HPO4)
- I-iron sulfide (Fe2S3)
- I-potassium bromide (KBr)
- I-calcium carbonate (CaCO3)
- I-sodium hypochlorite (NaClO)
- I-potassium sulfate (K2SO4)
- Manganese chloride (MnCl2)